Influence of the synergistic effect between Co–N–C and ceria on the catalytic performance for selective oxidation of ethylbenzene
Abstract
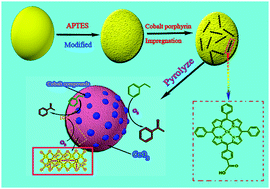
A series of catalysts, i.e. metal oxides (MO) such as CeO2, Fe2O3 and Al2O3 supported Co–N–C (Co–N–C/MO) were prepared by heating supported cobalt porphyrin in a N2 atmosphere. Among the Co–N–C/MO catalysts, Co–N–C/CeO2 shows a remarkable catalytic performance for ethylbenzene oxidation with ethylbenzene conversion of 33.1% and selectivity to acetophenone of 74.8%. In addition, the interaction between Co–N–C and supports was tentatively characterized by techniques such as XRD, HRTEM, XPS etc. According to XPS, the presence of the redox cycle between Ce3+ and Ce4+ in CeO2 facilitates the formation of cobalt ions in the high valence state and the Co–Nx sites, which are typically responsible for the high catalytic activity. The high performance benefits from the synergistic effect between Co–N–C and CeO2 and the well-dispersed Co-based sites.

 Please wait while we load your content...
Please wait while we load your content...