The structure and stability of reduced and oxidized mononuclear platinum species on nanostructured ceria from density functional modeling†
Abstract
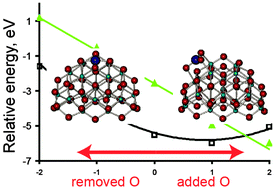
We report our results for the structure and relative stability of mononuclear platinum species on a ceria nanoparticle Ce21O42 depending on reduction or oxidation of the system. The most stable platinum species is Pt2+ at small {100} facets, where the ion is coordinated in a square-planar complex with four oxygen anions as ligands. Partial reduction of the system does not affect the state of platinum in this position but causes reduction of cerium ions. Atomic platinum species in all other modeled positions on the surface of the ceria nanoparticle are found to be in the oxidation state 0. Based on the calculated thermodynamic quantities we analyzed the formation of a preferable type of platinum species depending on the temperature and O2 pressure. Our thermodynamic model shows that the most stable species under standard conditions is PtO, while at the partial pressure of O2 below 100 Pa the stoichiometric complex Pt–Ce21O42 is formed. In both structures there is Pt2+ located in a square-planar complex. The characteristics of these two structures fit well the available EXAFS and XPS data. These structures are energetically stable with respect to sintering, while the agglomeration to platinum clusters is exothermic for the neutral mononuclear Pt species located at {111} facets.


 Please wait while we load your content...
Please wait while we load your content...