Viscosity minima in binary mixtures of ionic liquids + molecular solvents
Abstract
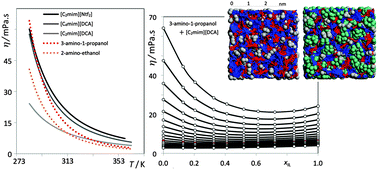
The viscosity (η) of four binary mixtures (ionic liquids plus molecular solvents, ILs+MSs) was measured in the 283.15 < T/K < 363.15 temperature range. Different IL/MS combinations were selected in such a way that the corresponding η(T) functions exhibit crossover temperatures at which both pure components present identical viscosity values. Consequently, most of the obtained mixture isotherms, η(x), exhibit clear viscosity minima in the studied T–x range. The results are interpreted using auxiliary molecular dynamics (MD) simulation data in order to correlate the observed η(T,x) trends with the interactions in each mixture, including the balance between electrostatic forces and hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...