The correlation of the binding mechanism of the polypyrrole–carbon capacitive interphase with electrochemical stability of the composite electrode
Abstract
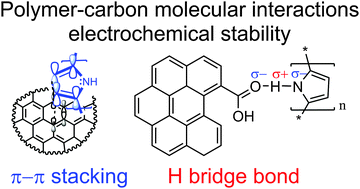
Carbon–polymer composites have great application potential in the field of organic batteries, capacitors, capacitive water desalination reactors and as the conductive platforms for electrochemical sensors. Although numerous studies have been carried out with respect to the synthesis, the optimization of composition, the carbon type and the morphology control, there is still a lack of understanding about which kind of intermolecular connection between carbon and polymer phases is preferential, and how the system should be designed to achieve the application demand of long-term electrochemical stability. Herein, we propose two model systems that employ the most well-known commercial carbons (SWCNTs and carbon black Vulcan XC72-R) to generate polypyrrole–C composites and validate the type of chemical bonding that is preferential to maintain electrochemical stability. In this work we used a simple oxidative polymerization of pyrrole and generated various formulations (with variable polymer content). Based on the surface XPS combined with bulk TGA-MS analysis we were able to evaluate the concentration and type of oxygen-containing functionalities, revealing a high oxygen content for the carbon black. It was further correlated with XPS analysis of the respective composites showed evidence of the electronic interaction called π–π* stacking between SWCNTs and PPy, and the binding energy shifts associated with the formation of hydrogen bridge bonds in the case of Vulcan XC-72R-PPy. Furthermore, the electrochemical stability of these model samples was investigated by AC impedance spectroscopy. The charge transfer resistance (Rct) was analyzed upon the oxidative potential, revealing SWCNT–PPy as an ultra-stable composite, even for the high polymer content (1 : 4 weight ratio of C–PPy). In contrast, the carbon black–PPy underwent rapid degradation in the whole composition range. The durability is associated with the type and strength of the polymer–carbon bonding as revealed by EIS impedance correlated with spectroscopic studies. The electronic interactions between SWCNTs and PPy result in superior stability while the carbon black–PPy, where the hydrogen bridge bonds are generated, is not stable under the same experimental conditions.

 Please wait while we load your content...
Please wait while we load your content...