A nucleus-coupled electron transfer mechanism for TiO2-catalyzed water splitting†
Abstract
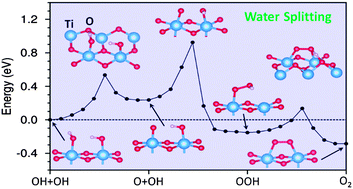
Based on first-principles calculations, we reveal that in the photocatalytic oxygen evolution reaction (OER) at the TiO2/water interface, the formation of an O–O bond always involves the anti-bonding σ2p* state elevated from the valence band into the conduction band of TiO2 regardless of a detailed reaction pathway. The role of photoholes is to deplete this anti-bonding state once it emerges into the band gap. The reaction barrier is thus determined by the onset where photoholes enter the reaction. This process represents a new reaction mechanism, termed nucleus-coupled electron transfer (NCET), where electron transfer is enabled by the movement of nuclei that promotes the reactive orbital to become the frontier orbital. The NCET mechanism for the OER is shown to exhibit an overall kinetic barrier surmountable at room temperature.

 Please wait while we load your content...
Please wait while we load your content...