The kinetics and mechanism of nanoconfined molten salt reactions: trimerization of potassium and rubidium dicyanamide
Abstract
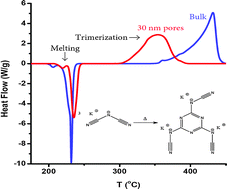
This study highlights the effect of the aggregate state of a reactant on the reaction kinetics under the conditions of nanoconfinement. Our previous work (Phys. Chem. Chem. Phys., 2014, 16, 11409) has demonstrated considerable deceleration of the solid state trimerization of sodium dicyanamide in organically modified silica nanopores. In the present study we use FTIR, NMR, pXRD, TGA and DSC to analyze the kinetics and mechanism of the liquid state trimerization of potassium and rubidium dicyanamide under similar conditions of nanoconfinement. It is found that nanoconfinement accelerates dramatically the kinetics of the liquid state trimerization, whereas it does not appear to affect the reaction mechanism. Kinetic analysis indicates that the acceleration is associated with an increase in the preexponential factor. Although nanoconfinement has the opposite effects on the respective kinetics of solid and liquid state trimerization, both effects are linked to a change in the preexponential factor. The results obtained are consistent with our hypothesis that the effects differ because nanoconfinement may promote disordering of the solid and ordering of the liquid reaction media.

 Please wait while we load your content...
Please wait while we load your content...