Hydrogen bonding motifs in a hydroxy-bisphosphonate moiety: revisiting the problem of hydrogen bond identification†
Abstract
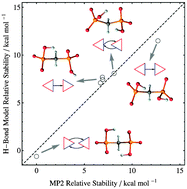
Bisphosphonates are important therapeutic agents in bone-related diseases and exhibit complex H-bonding networks. To assess the role of H-bonds in biophosphonate stability, a full conformational search was performed for methylenebisphosphonate (MBP) and 1-hydroxyethylidene-1,1-diphosphonate (HEDP) using the MP2 method in conjunction with the continuum solvation model. The most stable structures and their equilibrium populations were analyzed at two protonation states via assignment of H-bonding motifs to each conformer. Geometrical and topological approaches for the identification and characterization of H-bonds were compared with each other, and some of the important correlations between H-bond features were described over the entire conformational space of a hydroxy-bisphosphonate moiety. The topologically derived H-bond energy obtained from the local density of potential energy at bond critical points shows consistent correlations with other measures such as H-bond frequency shift. An inverse power form without an intercept predicts topological H-bond energies from hydrogen-acceptor distances with an RMS error of less than 1 kcal mol−1. The consistency of this measure was further checked by building a model that reasonably reproduces the relative stabilities of different conformers from their hydrogen-acceptor distances. In all systems, the predictions of this model are improved by the consideration of weak H-bonds that have no bond critical point.

 Please wait while we load your content...
Please wait while we load your content...