Molecular functionalization of silicene/Ag(111) by covalent bonds: a DFT study
Abstract
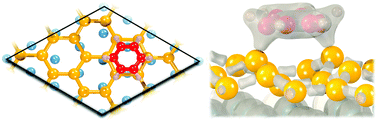
Among the 2D crystals, silicene, which forms sp2–sp3 bonds, is expected to present a higher reactivity than graphene, characterized by sp2 bonds only. However, silicene functionalization, in particular with organic molecules, remains an open question. By means of density functional theory, we study the adsorption of benzene, a model organic molecule, on (3 × 3) silicene on the (4 × 4) Ag(111) surface. Our calculations show that the dispersion interactions must be taken into account in order to describe this system properly. The adsorption energy is calculated by means of the semi-empirical dispersion-corrected density functional theory (DFT-D2) and the optB86b-vdW density functional. The charge density and electron localization function maps indicate that the molecule is chemisorbed on the silicene surface by means of two Si–C covalent bonds. In agreement with charge density difference calculations, two C–C double bonds are formed in the benzene molecule, which adopts a butterfly configuration. The silicene lattice is slightly deformed upon benzene adsorption, but the Si–Si distance remains the same as in bare silicene/Ag(111). Bader analysis shows a charge transfer from top Si atoms to both molecules and Ag substrates. Finally, we show that the covalent functionalization of silicene is possible.

 Please wait while we load your content...
Please wait while we load your content...