Thermally-induced chemical-order transitions in medium–large alloy nanoparticles predicted using a coarse-grained layer model
Abstract
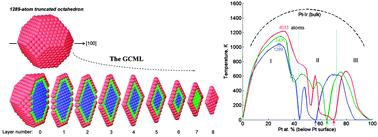
A new coarse-grained layer model (CGLM) for efficient computation of axially symmetric elemental equilibrium configurations in alloy nanoparticles (NPs) is introduced and applied to chemical-order transitions in Pt–Ir truncated octahedra (TOs) comprising up to tens of thousands of atoms. The model is based on adaptation of the free energy concentration expansion method (FCEM) using coordination-dependent bond-energy variations (CBEV) as input extracted from DFT-computed elemental bulk and surface energies. Thermally induced quite sharp transitions from low-T asymmetric quasi-Janus and quasi ball-and-cup configurations to symmetric multi-shells furnish unparalleled nanophase composite diagrams for 1289-, 2406- and 4033-atom NPs. At even higher temperatures entropic atomic mixing in the multi-shells gradually intensifies, as reflected in broad heat-capacity Schottky humps, which become sharper for much larger TOs (e.g., ∼10 nm, ∼30 000 atoms), due to transformation to solid-solution-like cores.
- This article is part of the themed collection: Recent advances in the chemical physics of nanoalloys

 Please wait while we load your content...
Please wait while we load your content...