Direct observation of the dealloying process of a platinum–yttrium nanoparticle fuel cell cathode and its oxygenated species during the oxygen reduction reaction†
Abstract
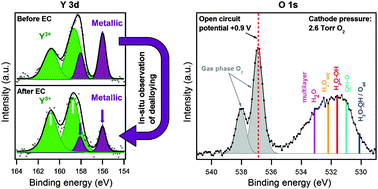
Size-selected 9 nm PtxY nanoparticles have recently shown an outstanding catalytic activity for the oxygen reduction reaction, representing a promising cathode catalyst for proton exchange membrane fuel cells (PEMFCs). Studying their electrochemical dealloying is a fundamental step towards the understanding of both their activity and stability. Herein, size-selected 9 nm PtxY nanoparticles have been deposited on the cathode side of a PEMFC specifically designed for in situ ambient pressure X-ray photoelectron spectroscopy (APXPS). The dealloying mechanism was followed in situ for the first time. It proceeds through the progressive oxidation of alloyed Y atoms, soon leading to the accumulation of Y3+ cations at the cathode. Acid leaching with sulfuric acid is capable of accelerating the dealloying process and removing these Y3+ cations which might cause long term degradation of the membrane. The use of APXPS under near operating conditions allowed observing the population of oxygenated surface species as a function of the electrochemical potential. Similar to the case of pure Pt nanoparticles, non-hydrated hydroxide plays a key role in the ORR catalytic process.
- This article is part of the themed collection: Recent advances in the chemical physics of nanoalloys


 Please wait while we load your content...
Please wait while we load your content...