The fate of phenothiazine-based redox shuttles in lithium-ion batteries†
Abstract
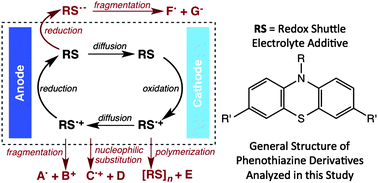
The stability and reactivity of the multiple oxidation states of aromatic compounds are critical to the performance of these species as additives and electrolytes in energy-storage applications. Both for the overcharge mitigation in ion-intercalation batteries and as electroactive species in redox flow batteries, neutral, radical-cation, and radical-anion species may be present during charging and discharging processes. Despite the wide range of compounds evaluated for both applications, the progress identifying stable materials has been slow, limited perhaps by the overall lack of analysis of the failure mechanism when a material is utilized in an energy-storage device. In this study, we examined the reactivity of phenothiazine derivatives, which have found interest as redox shuttles in lithium-ion battery applications. We explored the products of the reactions of neutral compounds in battery electrolytes and the products of radical cation formation using bulk electrolysis and coin cell cycling. Following the failure of each cell, the electrolytes were characterized to identify redox shuttle decomposition products. Based on these results, a set of decomposition mechanisms is proposed and further explored using experimental and theoretical approaches. The results highlight the necessity to fully characterize and understand the chemical degradation mechanisms of the redox species in order to develop new generations of electroactive materials.

 Please wait while we load your content...
Please wait while we load your content...