A class of rare antiferromagnetic metallic oxides: double perovskite AMn3V4O12 (A = Na+, Ca2+, and La3+) and the site-selective doping effect
Abstract
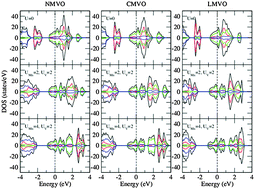
We have investigated the structural, electronic, and magnetic properties of A-site-ordered double-perovskite-structured oxides, AA′3B4O12 (A = Na, Ca, and La) with Mn and V at A′ and B sites, respectively, using first-principle calculations based on the density functional theory. Our calculation results show that the antiferromagnetic phase is the ground state for all the compounds. By changing the A-site ions from Na+ to Ca2+ and then to La3+, the transfer of charge between Mn and O ions was changed from 1.56 to 1.55 and then to 1.50, and that between the V and O ions changed from 2.01 to 1.95 and then to 1.93, revealing the cause for the unusual site-selective doping effect. Mn 3d electrons dominate the magnetic moment and are localized, with an intense hybridization with O 2p orbitals, which indicates that the magnetic exchange interaction between Mn ions is mediated through O and that the super exchange mechanism will take effect. These materials have a large one-electron bandwidth W, and the ratio of the on-site Coulomb repulsion U to W is less than the critical value (U/W)c, which leads to metallic behavior of AMn3V4O12. This is further evidenced by the large number of free electrons contributed by V at the Fermi surface. These calculations, in combination with the reported experimental data, prove that these double perovskites belong to the rare antiferromagnetic metallic oxides.

 Please wait while we load your content...
Please wait while we load your content...