Materials analyses and electrochemical impedance of implantable metal electrodes
Abstract
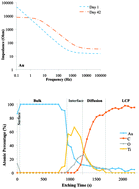
Implantable electrodes with high flexibility, high mechanical fixation and low electrochemical impedance are desirable for neuromuscular activation because they provide safe, effective and stable stimulation. In this paper, we report on detailed materials and electrical analyses of three metal implantable electrodes – gold (Au), platinum (Pt) and titanium (Ti) – using X-ray photoelectron spectroscopy (XPS), scanning acoustic microscopy, drop shape analysis and electrochemical impedance spectroscopy. We investigated the cause of changes in electrochemical impedance of long-term immersed Au, Pt and Ti electrodes on liquid crystal polymers (LCPs) in phosphate buffered saline (PBS). We analyzed the surface wettability, surface and interface defects and the elemental depth profile of the electrode-adhesion layers on the LCP. The impedance of the electrodes decreased at lower frequencies, but increased at higher frequencies compared with that of the short-term immersion. The increase of impedances was influenced by the oxidation of the electrode/adhesion-layers that affected the double layer capacitance behavior of the electrode/PBS. The oxidation of the adhesion layer for all the electrodes was confirmed by XPS. Alkali ions (sodium) were adsorbed on the Au and Pt surfaces, but diffused into the Ti electrode and LCPs. The Pt electrode showed a higher sensitivity to surface and interface defects than that of Ti and Au electrodes. These findings may be useful when designing electrodes for long-term implantable devices.

 Please wait while we load your content...
Please wait while we load your content...