A QCT study of the role of the symmetric and antisymmetric stretch mode excitations of methane in the O(3P) + CH4 (νi = 0, 1; i = 1, 3) reaction
Abstract
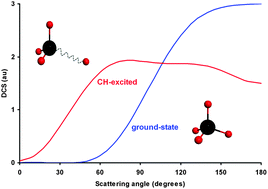
Quasi-classical trajectory calculations based on a full-dimensional analytical potential energy surface have been performed at different collision energies to analyze the role of symmetric (ν1 = 1) and antisymmetric (ν3 = 1) stretch modes of methane in reactivity and dynamics of the O(3P) + CH4 (νi = 0, 1; i = 1, 3) gas-phase reactions. Both CH stretch modes increase reactivity with respect to the methane vibrational ground-state by factors between 1.5 and 3. Additionally, the ν1 = 1 mode is slightly more reactive than the ν3 = 1 mode by factors between 1.4 and 1.1 depending on the collision energy. Both stretch modes give similar pictures of OH product vibrational and angular distributions. The former finding shows inverted OH (0, 1) vibrational population, discarding mode selectivity, and the latter shows a shift of the scattering angle from backward to sideways with the vibrational excitation and therefore a change in the mechanism. For the dynamic properties analyzed, the theoretical results for the ν3 = 1 mode reproduce the experimental evidence, while those for the ν1 = 1 mode await confirmation.

 Please wait while we load your content...
Please wait while we load your content...