Kinetics of aggregation in liquids with dispersed nanoparticles†
Abstract
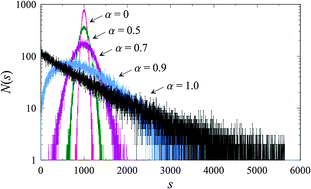
The process of attaching molecules of liquid media by dispersed nanoparticles is modeled and numerically studied. The growth rate of the resulting nanoparticle-induced aggregates is determined by assuming the preferential attachment rule according to which the effectiveness of the connection of a new molecular unit to aggregates is determined by their size. It is shown that, depending on a specific functional form of the growth rate, the size distribution of aggregates can display very different shapes, including various multimodal structures. This can explain experimentally obtained complex size distributions of inhomogeneous aggregates appearing as a consequence of the adsorption of molecules by nanoparticles or as a consequence of the self-assembling of active dispersants on surfaces of nanoparticles. The time evolution and the stationarity of the size distribution are also analyzed, gaining an insight into the long-time behavior of systems with dispersed nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...