From charge transfer to electron transfer in halogen-bonded complexes of electrophilic bromocarbons with halide anions†
Abstract
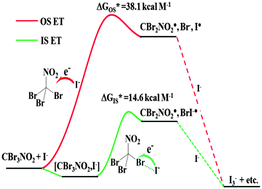
Experimental and computational studies of the halogen-bonded complexes, [R–Br, X−], of bromosubstituted electrophiles, R–Br, and halide anions, X−, revealed that decrease of a gap between the frontier orbitals of interacting species led to reduction of the energy of the optical charge-transfer transition and to increase in the ground-state charge transfer (X− → R–Br) in their associates. These variations were accompanied by weakening of the intramolecular, C–Br, and strengthening of the intermolecular, Br⋯X−, bonds. In the limit of the strongest electron donor–acceptor pairs, formation of the halogen-bonded complexes was followed by the oxidation of iodide to triiodide, which took place despite the fact that the I− → R–Br electron-transfer step was highly endergonic and the calculated outer-sphere rate constant was negligibly small. However, the calculated barrier for the inner-sphere electron transfer accompanied by the halogen transfer, R–Br⋯I− → R˙⋯Br–I−˙, was nearly 24 kcal mol−1 lower as compared to that calculated for the outer-sphere process and the rate constant of such reaction was consistent with the experimental kinetics. A dramatic decrease of the electron-transfer barriers (leading to 18-orders of magnitude increase of the rate constant) was related to the strong electronic coupling of the donor and acceptor within the halogen-bonded precursor complex, as well as to the lower solvent reorganization energy and the successor-complex stabilization.

 Please wait while we load your content...
Please wait while we load your content...