Theoretical examination of solvent and R group dependence in gold thiolate nanoparticle synthesis
Abstract
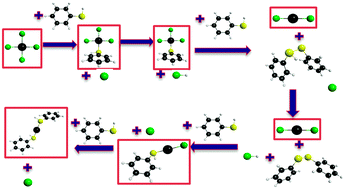
The growth of gold thiolate nanoparticles can be affected by the solvent and the R group on the ligand. In this work, the difference between methanol and benzene solvents as well as the effect of alkyl (methyl) and aromatic (phenyl) thiols on the reaction energies and barrier heights is investigated theoretically. Density functional theory (DFT) calculations using the BP86 functional and a triple ζ polarized basis set show that the overall reaction favors methylthiol over phenylthiol with reaction energies of −0.54 and −0.39 eV in methanol, respectively. At the same level of theory, the methanol solvent is favored over the benzene solvent for reactions forming ions; in benzene, the overall reaction energies for methylthiol and phenylthiol reacting with AuCl4− to form Au(HSR)2+ are 0.37 eV and 0.44 eV, respectively. Methylthiol in methanol also has the lowest barrier heights at about 0.3 eV, whereas phenylthiol has barrier heights around 0.4 eV. Barrier heights in benzene are significantly larger than those in methanol.
- This article is part of the themed collection: Size Selected Clusters and Particles: From Physical Chemistry to Catalysis

 Please wait while we load your content...
Please wait while we load your content...