Water transport in the nano-pore of the calcium silicate phase: reactivity, structure and dynamics
Abstract
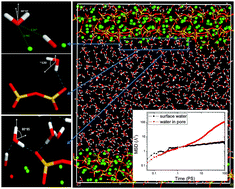
Reactive force field molecular dynamics was utilized to simulate the reactivity, structure and dynamics of water molecules confined in calcium–silicate–hydrate (C–S–H) nano-pores of 4.5 nm width. Due to the highly reactive C–S–H surface, hydrolytic reactions occur in the solid–liquid interfacial zone, and partially surface adsorbed water molecules transforming into the Si–OH and Ca–OH groups are strongly embedded in the C–S–H structure. Due to the electronic charge difference, the silicate and calcium hydroxyl groups have binomial distributions of the dipolar moment and water orientation. While Ca–OH contributes to the Ow-downward orientation, the ONB atoms in the silicate chains prefer to accept H-bonds from the surface water molecules. Furthermore, the defective silicate chains and solvated Caw atoms near the surface contribute to the glassy nature of the surface water molecules, with large packing density, pronounced orientation preference, and distorted organization. The stable H-bonds connected with the Ca–OH and Si–OH groups also restrict the mobility of the surface water molecules. The significant reduction of the diffusion coefficient matches well with the experimental results obtained by NMR, QENS and PCFR techniques. Upon increasing the distance from the channel, the structural and dynamic behavior of the water molecules varies and gradually translates into bulk water properties at distances of 10–15 Å from the liquid–solid interface.

 Please wait while we load your content...
Please wait while we load your content...