Fundamental frequency from classical molecular dynamics†
Abstract
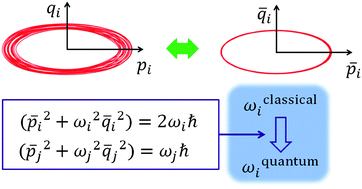
We give a theoretical validation for calculating fundamental frequencies of a molecule from classical molecular dynamics (MD) when its anharmonicity is small enough to be treated by perturbation theory. We specifically give concrete answers to the following questions: (1) What is the appropriate initial condition of classical MD to calculate the fundamental frequency? (2) From that condition, how accurately can we extract fundamental frequencies of a molecule? (3) What is the benefit of using ab initio MD for frequency calculations? Our analytical approaches to those questions are classical and quantum normal form theories. As numerical examples we perform two types of MD to calculate fundamental frequencies of H2O with MP2/aug-cc-pVTZ: one is based on the quartic force field and the other one is direct ab initio MD, where the potential energies and the gradients are calculated on the fly. From those calculations, we show comparisons of the frequencies from MD with the post vibrational self-consistent field calculations, second- and fourth-order perturbation theories, and experiments. We also apply direct ab initio MD to frequency calculations of C–H vibrational modes of tetracene and naphthalene. We conclude that MD can give the same accuracy in fundamental frequency calculation as second-order perturbation theory but the computational cost is lower for large molecules.

 Please wait while we load your content...
Please wait while we load your content...