Charge transfer to solvent dynamics in iodide aqueous solution studied at ionization threshold†
Abstract
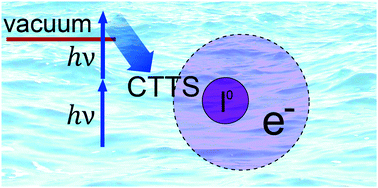
We explore the early-time electronic relaxation in NaI aqueous solution exposed to a short UV laser pulse. Rather than initiating the charge transfer reaction by resonant photoexcitation of iodide, in the present time-resolved photoelectron spectroscopy study the charge-transfer-to-solvent (CTTS) states are populated via electronic excitation above the vacuum level. By analyzing the temporal evolution of electron yields from ionization of two transient species, assigned to CTTS and its first excited state, we determine both their ultrafast population and relaxation dynamics. Comparison with resonant-excitation studies shows that the highly excited initial states exhibit similar relaxation characteristics as found for resonant excitation. Implications for structure and dynamical response of the hydration cage are discussed.

 Please wait while we load your content...
Please wait while we load your content...