Gaussian analysis of Raman spectroscopy of acetic acid reveals a significant amount of monomers that effectively cooperate with hydrogen bonded linear chains†
Abstract
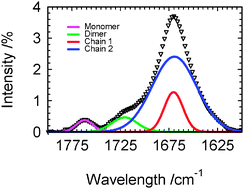
Gaussian analysis of Raman spectroscopy reveals three significant structures in the liquid acetic acid (AA): linear chains involving C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and O–H⋯O
C and O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding, cyclic dimers and dissociated monomers that can effectively cooperate with hydrogen bonded stacks of linear AA or polymer chains.
C hydrogen bonding, cyclic dimers and dissociated monomers that can effectively cooperate with hydrogen bonded stacks of linear AA or polymer chains.

 Please wait while we load your content...
Please wait while we load your content...