Promoting alkali and alkaline-earth metals on MgO for enhancing CO2 capture by first-principles calculations
Abstract
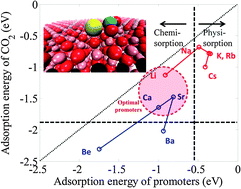
Developing next-generation solid sorbents to improve the economy of pre- and post-combustion carbon capture processes has been challenging for many researchers. Magnesium oxide (MgO) is a promising sorbent because of its moderate sorption–desorption temperature and low heat of sorption. However, its low sorption capacity and thermal instability need to be improved. Various metal-promoted MgO sorbents have been experimentally developed to enhance the CO2 sorption capacities. Nevertheless, rigorous computational studies to screen an optimal metal promoter have been limited to date. We conducted first-principles calculations to select metal promoters of MgO sorbents. Five alkali (Li-, Na-, K-, Rb-, and Cs-) and 4 alkaline earth metals (Be-, Ca-, Sr-, and Ba-) were chosen as a set of promoters. Compared with the CO2 adsorption energy on pure MgO, the adsorption energy on the metal-promoted MgO sorbents is higher, except for the Na-promoter, which indicates that metal promotion on MgO is an efficient approach to enhance the sorption capacities. Based on the stabilized binding of promoters on the MgO surface and the regenerability of sorbents, Li, Ca, and Sr were identified as adequate promoters among the 9 metals on the basis of PW91/GGA augmented with DFT+D2. The adsorption energies of CO2 on metal-promoted MgO sorbents for Li, Ca, and Sr atoms are −1.13, −1.68, and −1.48 eV, respectively.

 Please wait while we load your content...
Please wait while we load your content...