Why does the IR spectrum of hydroxide stretching vibration weaken with increase in hydration?†
Abstract
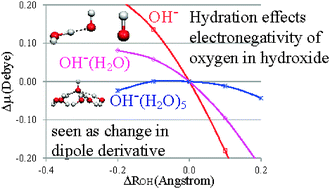
The infrared absorption intensity of hydroxide stretching vibration is strong in the gas phase, however it becomes weak in the aqueous phase. To provide an understanding of this relationship, we performed theoretical studies on OH−(H2O)n, n = 0–5 clusters, with the water molecules bound to the oxygen edge of the hydroxide. We found that with the increase in n, the infrared intensity of hydroxide stretching vibration decreases. This is rationalized by the positive potential that the water molecules exert on the oxygen edge of the hydroxide. Directional hydrogen bonding by the water molecules stabilizes the oxygen atomic orbitals in hydroxide, thus decreasing the electron-migration from oxygen 2pσ to hydrogen in hydroxide. These characteristics cause the dipole moment derivative of the hydroxide stretching vibration to be minimal, resulting in a weaker infrared absorption intensity for large n. In addition, we showed that this hydration effect can also be observed through the successive increase in the vertical electron detachment energy as a function of n. Lastly, we discuss the implications of this study on the dangling hydroxide at the air–water interface.

 Please wait while we load your content...
Please wait while we load your content...