The dependence of surface tension on surface properties of ionic surfactant solution and the effects of counter-ions therein
Abstract
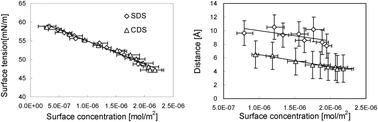
In the present paper, we aim to investigate the dependence of surface tension on the surface properties and reveal the counter-ion effects on the adsorption of ionic surfactants on the solution surface. The surface tension, surface excess and surface concentration (defined as the amount of surfactant adsorbed in the surface phase divided by the surface area) of two anionic surfactants, namely dodecyl sulfate sodium and dodecyl sulfate caesium, dissolved in non-aqueous polar solvent formamide have been separately measured at 6 °C through independent experiments. Then, the correlation of surface tension with surface concentration and that of surface tension with surface excess is inspected in detail. It was found that there is a linear relationship between the surface tension and the surface concentration for the pure solutions of each surfactant, but their surface tension and surface excess cannot be correlated linearly. It is striking that the same surface tension–surface concentration linearity holds for two different surfactants, although they have apparently distinct counter-ions. Based on this finding, it is derived that the surface tension is decided by surface concentration of the surface active ions. After analyzing the surface structure, it is concluded that the counter-ions affect the surface tension indirectly through modifying the adsorption amount of the surface active ions in the surface layer.

 Please wait while we load your content...
Please wait while we load your content...