The positions of inner hydroxide groups and aluminium ions in exfoliated kaolinite as indicators of the external chemical environment†
Abstract
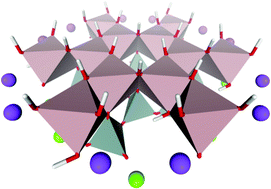
Kaolinite as a remarkable industrial raw material has notable structural features despite its simple chemical composition (Al2O3·2SiO2·2H2O). We report here a systematic development of a coordination chemical model for the [6Al-6(OH)] honeycomb-like unit of kaolinite's octahedral sheet, which was proposed to be the adsorption site for small molecules from earlier studies. The coordination environment of the Al3+ ions was completed with outer sphere groups from both octahedral and tetrahedral sheets. Dangling bonds were terminated by additional Al3+ and Si4+ ions with hydroxide and oxide groups from the second coordination sphere versus simple protonation. A cage of Na+ and Mg2+ ions rendered the computational model to be charge neutral. In this exfoliated kaolinite model, the inner hydroxide groups and the adjacent Al3+ ions have compositionally the most complete environments with respect to the crystal structure. Thus, their atomic positions were used as a benchmark for the level of theory dependence of the optimized structures. We evaluated the performance of a representative set of density functionals, basis sets, point-charges, identified pitfalls and caveats. Importantly, the structural changes during optimization of periodic and cluster models suggest pliability for the exfoliated kaolinite layers, which is influenced by the external chemical environment.

 Please wait while we load your content...
Please wait while we load your content...