A study of the ice–water interface using the TIP4P/2005 water model
Abstract
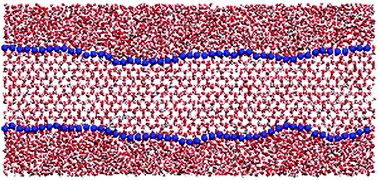
In this work we study the ice–water interface under coexistence conditions by means of molecular simulations using the TIP4P/2005 water model. Following the methodology proposed by Hoyt and co-workers [J. J. Hoyt, M. Asta and A. Karma, Phys. Rev. Lett., 2001, 86, 5530] we measure the interfacial free energy of ice with liquid water by analysing the spectrum of capillary fluctuations of the interface. We get an orientationally averaged interfacial free energy of 27(2) mN m−1, in good agreement with a recent estimate obtained from simulation data of the size of critical clusters [E. Sanz, C. Vega, J. R. Espinosa, R. Caballero-Bernal, J. L. F. Abascal and C. Valeriani, J. Am. Chem. Soc., 2013, 135, 15008]. We also estimate the interfacial free energy of different planes and obtain 27(2), 28(2) and 28(2) mN m−1 for the basal, the primary prismatic and the secondary prismatic planes respectively. Finally, we inspect the structure of the interface and find that its thickness is approximately 4–5 molecular diameters. Moreover, we find that when the basal plane is exposed to the fluid the interface alternates regions of cubic ice with regions of hexagonal ice.

 Please wait while we load your content...
Please wait while we load your content...