Hydrogen-induced high-temperature segregation in palladium silver membranes
Abstract
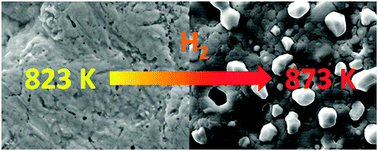
Higher operation temperatures benefit H2 permeability and selectivity of metal membranes and they are interesting for e.g. water gas shift and steam reforming in membrane reactors. Hence the behaviour of PdAg–ceramic composite membranes has been investigated between 823 K and 923 K. The H2 flux of membranes with less than 10 μm thick alloy layers decreased continuously with time during operation under H2 at 873 K and above. This was accompanied by a steady increase of the activation energy for H2 permeation and the growth of Ag-depleted crystallites on the membrane surface. All phenomena could be reversed through annealing under N2 at 923 K. The textural and permeability changes are consistent with a segregation mechanism starting with metal sublimation from hydrogenated PdAg layers and subsequent metal resublimation. This implies an enhancement of the yet unknown metal activities in PdAg hydride phases over metallic PdAg alloys. Ramifications for application of thin-layered, supported PdAg membranes for H2 separation above 823 K are discussed.

 Please wait while we load your content...
Please wait while we load your content...