Probing the kinetic energy-release dynamics of H-atom products from the gas-phase reaction of O(3P) with vinyl radical C2H3
Abstract
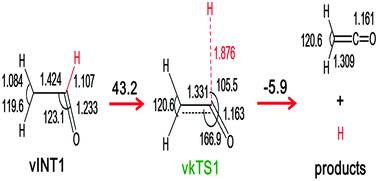
The gas-phase radical–radical reaction dynamics of ground-state atomic oxygen O(3P) with vinyl radicals C2H3 has been studied by combining the results of vacuum-ultraviolet laser-induced fluorescence spectroscopy in a crossed beam configuration with ab initio calculations. The two radical reactants O(3P) and C2H3 were produced by photolysis of NO2 and supersonic flash pyrolysis of C2H3I, respectively. Doppler profile analysis of the kinetic energy release of the nascent H-atom products from the title reaction O(3P) + C2H3 → H(2S) + CH2CO (ketene) revealed that the average translational energy of the products and the average fraction of the total available energy were 7.03 ± 0.30 kcal mol−1 and 7.2%. The empirical data combined with CBS-QB3 level ab initio theory and statistical calculations demonstrated that the title oxygen–hydrogen exchange reaction is a major reaction channel, through an addition–elimination mechanism involving the formation of a short-lived, dynamical complex on the doublet potential energy surface. On the basis of systematic comparison with several exchange reactions of hydrocarbon radicals, the observed kinetic energy release can be explained in terms of the weak impulse at the moment of decomposition in the loose transition state with a product-like geometry and a small reverse barrier along the exit channel.

 Please wait while we load your content...
Please wait while we load your content...