Improving water splitting performance of Cu2O through a synergistic “two-way transfer” process of Cu and graphene†
Abstract
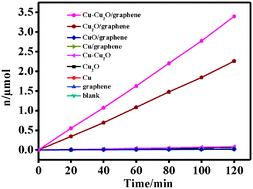
H2 evolution catalysis has drawn great consideration and effective separation and delivery of the photoelectrons are particularly crucial during the whole process. In this paper, we fabricate porous Cu–Cu2O–graphene nanocomposites via a simple reflux synthesis route, which possess porous structure and excellent catalytic performance for water splitting. With Cu species being added into Cu2O–graphene, the resultant catalyst exhibits improved activity for H2 evolution reaction as compared to Cu2O, Cu–Cu2O and Cu2O–graphene, indicating excellent catalytic performance and potential practical use. We attribute this performance to the synergistic effect of Cu component and graphene, which features: (i) a broader range of light absorption; (ii) faster electron transfer; and (iii) lower recombination possibility of photogenerated electrons and holes. We believe that the Cu species and graphene both contribute greatly to this catalysis process, in which Cu can cooperate with graphene support to extract electrons and pass them to the Pt cocatalyst to form a “two-way transfer” process. It is also believed that this strategy can be extended to other catalysts based on Cu–Cu2O–graphene composites.

 Please wait while we load your content...
Please wait while we load your content...