Cu/ZnO nanocatalysts in response to environmental conditions: surface morphology, electronic structure, redox state and CO2 activation†
Abstract
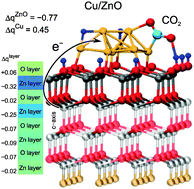
Methanol synthesis is one of the landmarks of heterogeneous catalysis due to the great industrial significance of methanol as a clean liquid fuel and as a raw material for industry. Understanding in atomistic detail the properties of the underlying metal/oxide catalyst materials as a function of temperature and composition of the reactive gas phase is of utmost importance in order to eventually improve the production process. By performing extensive density functional theory based slab calculations in combination with a thermodynamic formalism we establish an atomistic understanding of gas phase-induced changes of surface morphology, redox properties and reactivity of ZnO supported Cu nanocatalysts. Extending our recent insights [Phys. Rev. Lett., 2013, 110, 086108], we explore surface stabilization mechanisms and site-dependent redox states of both catalyst components as well as the pronounced electronic charge transfer processes across the metal–support interface. Moreover, ab initio molecular dynamics simulations unveil the vital role played by dynamical shape fluctuations of the deposited Cu8 cluster. The pronounced structural flexibility of the metal nanoparticle is found to enhance CO2 activation over Cu8 at the elevated temperature conditions of the industrial methanol synthesis process, in addition to activation of CO2via electronic charge transfer from the ZnO support.

 Please wait while we load your content...
Please wait while we load your content...