Flue gas CO2 mineralization using thermally activated serpentine: from single- to double-step carbonation†
Abstract
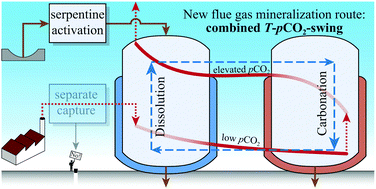
Carbon dioxide capture and utilization by mineralization seeks to combine greenhouse gas emission control with the production of value-added materials in the form of solid carbonates. This experimental work demonstrates that the world's most abundant mineralization precursor, the magnesium (Mg) silicate serpentine, in its thermally activated, partially dehydroxylated form can be carbonated without the use of chemical additives at process temperatures (T) below 90 °C and CO2 partial pressures (pCO2) below 1 bar. A first series of single-step batch experiments was performed varying the temperature and slurry density to systematically assess the precipitation regime of the relevant Mg-carbonates and the fate of silicon (Si) species in solution. The results suggested that the reaction progress was hindered by a passivating layer of re-precipitated silica or quartz, as well as by equilibrium limitations. Concurrent grinding proved effective in tackling the former problem. A double-step strategy proved successful in addressing the latter problem by controlling the pH of the solution. This is achieved by continuously removing the Mg from the dissolution reactor and letting it precipitate at a higher T and a lower pCO2 in a separate reactor, thus yielding a combined T–pCO2-swing—the working principle of a new flue gas mineralization route is presented herein. Simulations and experiments of the different individual steps of the process are reported, in order to make an assessment of its feasibility.


 Please wait while we load your content...
Please wait while we load your content...