Low-energy-electron induced permanently reactive CO2 molecules
Abstract
Ab initio quantum chemical studies show that a very weak molecular complexation of CO2 with a dipolar molecule is able to suppress the autoionization of the electron from its transient negative ion states. Since the autoionization is suppressed, the transient negative ion can efficiently relax its geometry to form the reductively activated CO2 moiety. Unlike the reductively activated isolated CO2 molecules, which are deactivated immediately due to their thermodynamic metastability, the reductively activated CO2 moieties of the weak molecular complexes are infinitely long-lived and, hence, permanently reactive.
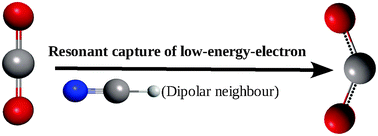

 Please wait while we load your content...
Please wait while we load your content...