Predicting bond strength from a single Hartree–Fock ground state using the localized pair model
Abstract
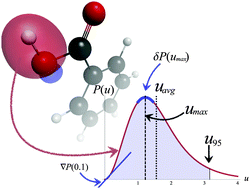
We present an application of the recently introduced Localized Pair Model (LPM) [Z. A. Zielinksi and J. K. Pearson, Comput. Theor. Chem., 2013, 1003, 7990] to characterize and quantify properties of the chemical bond in a series of substituted benzoic acid molecules. By computing interelectronic distribution functions for doubly-occupied Edmiston–Ruedenberg localized molecular orbitals (LMOs), we show that chemically intuitive electron pairs may be uniquely classified and bond strength may be predicted with remarkable accuracy. Specifically, the HF/u6-311G(d,p) level (where u denotes a complete uncontraction of the basis set) is used to generate the relevant LMOs and their respective interelectronic distribution functions can be linearly correlated to the well-known Hammett σp or σm parameters with near-unity correlation coefficients.

 Please wait while we load your content...
Please wait while we load your content...