Tetracyano isoindigo small molecules and their use in n-channel organic field-effect transistors†
Abstract
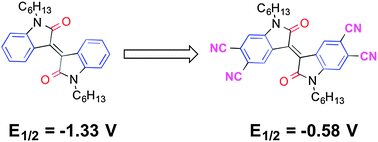
N,N′-Dihexyl-6,6′-dicyanoisoindigo, N,N′-didecyl-5,5′,6,6′-tetracyanoisoindigo, N,N′-dihexyl-5,5′,6,6′-tetracyanoisoindigo, and N,N′-dihexyl-5,5′,6,6′-tetracyanothienoisoindigo have been synthesised in moderate yields by the reaction of corresponding di and tetrabromo species with CuCN, with microwave heating leading to higher yields and fewer side products for the tetrasubstituted species. Di- and tetracyano substitution anodically shifts the molecular reduction potential relative to the unsubstituted cores by ca. 0.4 and 0.8 V, respectively, with the resultant values for the tetracyano derivatives (−0.58 to −0.67 V vs. FeCp2+/0) suggesting the possibility of air-stable electron transport. All the synthesised cyano derivatives operate in n-channel OFETs, while the tetrabromothienoisoindigo derivative functions in a p-channel transistor. The tetracyanothienoisoindigo derivative exhibits the highest field-effect electron mobility values – up to 0.04 and 0.09 cm2 V−1 s−1 in spin-coated and inkjet-printed devices respectively – and OFETs incorporating this compound have been shown to operate in air without significant degradation of their mobility values in the saturation regime.
- This article is part of the themed collection: Organic Field Effect Transistors

 Please wait while we load your content...
Please wait while we load your content...