Substitution of a hydroxamic acid anchor into the MK-2 dye for enhanced photovoltaic performance and water stability in a DSSC†
Abstract
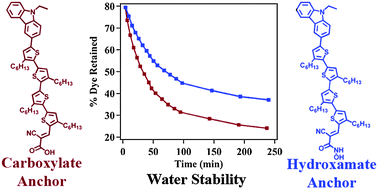
An efficient synthetic protocol to functionalize the cyanoacrylic acid anchoring group of commercially available MK-2 dye with a highly water-stable hydroxamate anchoring group is described. Extensive characterization of this hydroxamate-modified dye (MK-2HA) reveals that the modification does not affect its favorable optoelectronic properties. Dye-sensitized solar cells (DSSCs) prepared with the MK-2HA dye attain improved efficiency (6.9%), relative to analogously prepared devices with commercial MK-2 and N719 dyes. The hydroxamate anchoring group also contributes to significantly increased water stability, with a decrease in the rate constant for dye desorption of MK-2HA relative to MK-2 in the presence of water by as much as 37.5%. In addition, the hydroxamate-anchored dye undergoes essentially no loss in DSSC efficiency and the external quantum efficiency improves when up to 20% water is purposefully added to the electrolyte. In contrast, devices prepared with the commercial dye suffer a 50% decline in efficiency under identical conditions, with a concomitant decrease in external quantum efficiency. Collectively, our results indicate that covalent functionalization of organic dyes with hydroxamate anchoring groups is a simple and efficient approach to improving the water stability of the dye–semiconductor interface and overall device durability.

 Please wait while we load your content...
Please wait while we load your content...