Insight into the promiscuous activity of human carbonic anhydrase against the cyanic acid substrate from a combined QM and QM/MM investigation†
Abstract
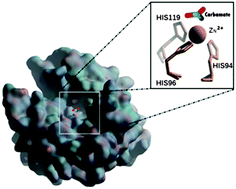
The promiscuous activity of human carbonic anhydrase (hCAII) against a non-physiological cyanic acid substrate has been investigated by using a combined QM and QM/MM level of theory. Results show that the hCAII is able to hydrate the cyanic acid by a reaction mechanism similar to that of the CO2 native substrate. The energy barrier for the nucleophilic attack is found to be 15.6 and 4.3 kcal mol−1 at QM and QM/MM levels, respectively. This result underlines the importance of taking into account the surrounding residues around the active site in the presence of the substrate having small molecular sizes. The carbamate is strongly stabilized with respect to the bicarbonate of the native substrate indicating a more difficult release of the reaction product.

 Please wait while we load your content...
Please wait while we load your content...