Reversible addition of the OH radical to p-cymene in the gas phase: multiple adduct formation. Part 2
Abstract
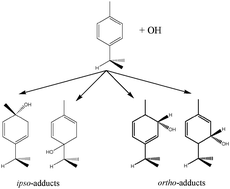
A flash photolysis-resonance fluorescence (FP-RF) system was used to study the p-cymene (PC) + OH reaction at temperatures between 299 and 349 K in helium. Triexponential functions were fitted to groups of observed OH decay curves according to a model considering a reversible addition to form two adducts as thermolabile reservoirs of OH. Compared to Part 1 of this paper, consideration of a second adduct strongly improved the fits to our measurements, and the rate constants for the major pathways were optimized between 299 and 349 K. The Arrhenius expression for the rate constant of the sum of OH addition and H-atom abstraction pathways was found to be kOH = 1.9 × 10−12 exp[(610 ± 210) K/T] cm3 s−1. Rate constants of unimolecular decomposition reactions of the adducts were similar to other aromatic compounds with the following Arrhenius expressions: 1 × 1012 exp[(−7600 ± 800) K/T] s−1 for adduct 1 and 4 × 1011 exp[(−8000 ± 300) K/T] s−1 for adduct 2. Adduct yields increased and decreased with temperature for adduct 1 and 2, respectively, but were similar (∼0.4) around room temperature. Equilibrium constants yielded values for reaction enthalpies and entropies of adduct formations. While for one adduct reasonable agreement was obtained with theoretical predictions, there were significant deviations for the other adduct. This indicates the presence of more than two adduct isomers that were not accounted for in the reaction model. Quantum chemical calculations (DFT M06-2X/6-31G(d,p)) and RRKM kinetics were employed with the aim of clarifying the mechanism of the OH addition to PC. These calculations show that formation of adducts with OH in ortho positions to the isopropyl and methyl substituents is predominant (55% and 24%) to those with OH in ipso positions (21% and 3%). A large fraction (>90%) of the ipso-C3H7 adduct is predicted to react by dealkylation forming p-cresol (in the absence of oxygen) and isopropyl radicals. These theoretical results agree well with the interpretation of the experimental results showing that the two ortho adducts (which appeared as OH reservoirs in the experiment) have been observed.

 Please wait while we load your content...
Please wait while we load your content...