Dynamic propensity as an indicator of heterogeneity in room-temperature ionic liquids
Abstract
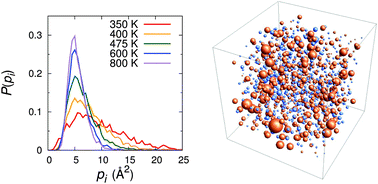
We investigate the dynamic propensity in a coarse-grained model of a room-temperature ionic liquid via molecular dynamics simulations. Dynamic propensity is defined as the average of squared displacements for each ion during a given time interval over the isoconfigurational ensemble. As the temperature is lowered, distributions of the dynamic propensity develop fat tails at high values, indicating the presence of dynamic heterogeneity in the system. The increase in the heterogeneity for the cation is more evident than that for the anion, and a high propensity exhibits a large variance in the isoconfigurational ensemble, implying that dynamic propensity is related to ions' motions at a large length scale, rather than a direct measure of the individual ion dynamics. In addition, large non-Gaussian parameters observed for small dynamic propensities reveal intermittent dynamical behaviors of ions. In order to reveal the origin of the dynamic heterogeneity in a room-temperature ionic liquid, a possible correlation between the mobility and dynamic propensity is further probed. It is observed that spatial distributions of the dynamic propensity coincide with those of the mobility. The results suggest a possible connection between the structure and heterogeneous dynamics on large length scales.

 Please wait while we load your content...
Please wait while we load your content...