Ultrafast Z → E photoisomerisation of structurally modified furylfulgides†
Abstract
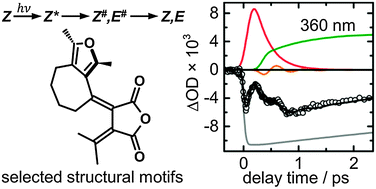
Femtosecond broadband transient absorption spectroscopy has been used in a comparative study of the ultrafast photo-induced Z → E isomerisation reactions of four photochromic furylfulgides with selected structural motifs in n-hexane as solvent. The results show that all studied Z-fulgides exhibit fast and direct processes along barrierless excited-state pathways involving a conical intersection (CI) between the S1 and S0 electronic states. The excited-state lifetimes range from τ1 = 0.18 ps for the methyl derivative to τ1 = 0.32 ps for the benzofurylfulgide. The impulsive rise of the absorption by vibrationally hot Z- and E-isomers back in the electronic ground state following electronic deactivation and isomerisation indicates that the initially prepared wave packet persists even after passage of the CI. Furthermore, the results provide qualitative evidence for a quickly dephasing vibrational coherence in the electronic ground state. In contrast to the significant changes observed for the corresponding E- and C-isomers [Renth et al., Int. Rev. Phys. Chem., 2013, 32, 1–38], the excited-state dynamics of the Z-isomers is not affected by varied sterical hindrance from methyl and isopropyl substituents at the central hexatriene unit, or by intramolecular bridging, and remains unaltered upon extension of the π-electron system in a benzannulated furyl fulgide.

 Please wait while we load your content...
Please wait while we load your content...