Ion-pair formation in aqueous strontium chloride and strontium hydroxide solutions under hydrothermal conditions by AC conductivity measurements
Abstract
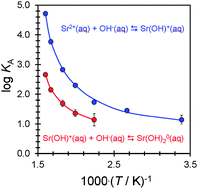
Frequency-dependent electrical conductivities of solutions of aqueous strontium hydroxide and strontium chloride have been measured from T = 295 K to T = 625 K at p = 20 MPa, over a very wide range of ionic strength (3 × 10−5 to 0.2 mol kg−1), using a high-precision flow AC conductivity instrument. Experimental values for the concentration-dependent equivalent conductivity, Λ, of the two electrolytes were fitted with the Turq–Blum–Bernard–Kunz (“TBBK”) ionic conductivity model, to determine ionic association constants, KA,m. The TBBK fits yielded statistically significant formation constants for the species SrOH+ and SrCl+ at all temperatures, and for Sr(OH)02 and SrCl02 at temperatures above 446 K. The first and second stepwise association constants for the ion pairs followed the order KA1(SrOH+) > KA1(SrCl+) > KA2[Sr(OH)02] > KA2[SrCl02], consistent with long-range solvent polarization effects associated with the lower static dielectric constant and high compressibility of water at elevated temperatures. The stepwise association constants to form SrCl+ agree with previously reported values for CaCl+ to within the combined experimental error at high temperatures and, at temperatures below ∼375 K, the values of log10 KA1 for strontium are lower than those for calcium by up to ∼0.3–0.4 units. The association constants for the species SrOH+ and Sr(OH)02 are the first accurate values to be reported for hydroxide ion pairs with any divalent cation under these conditions.

 Please wait while we load your content...
Please wait while we load your content...