Photo-physical properties of 2-(1-ethynylpyrene)-adenosine: influence of hydrogen bonding on excited state properties†
Abstract
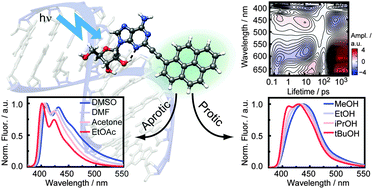
The photo-physical properties of 2-(1-ethynylpyrene)-adenosine (PyA), a fluorescent probe for RNA dynamics, were examined by solvation studies. The excited-state dynamics display the influence of the vicinity on the spectral features. Combining improved transient absorption and streak camera measurements along with a new analysis method provide a detailed molecular picture of the photophysics. After intramolecular vibrational energy redistribution (IVR), two distinct states are observed. Solvent class (protic/aprotic) and permittivity strongly affect the properties of these states and their population ratio. As a result their emission spectrum is altered, while the fluorescence quantum yield and the overall lifetime remain nearly unchanged. Consequently, the hitherto existing model of the photophysics is herein refined and extended. The findings can serve as basis for improving the information content of measurements with PyA as a label in RNA.

 Please wait while we load your content...
Please wait while we load your content...