Significance of β-dehydrogenation in ethanol electro-oxidation on platinum doped with Ru, Rh, Pd, Os and Ir
Abstract
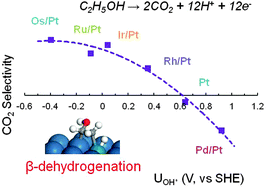
In the exploration of highly efficient direct ethanol fuel cells (DEFCs), how to promote the CO2 selectivity is a key issue which remains to be solved. Some advances have been made, for example, using bimetallic electrocatalysts, Rh has been found to be an efficient additive to platinum to obtain high CO2 selectivity experimentally. In this work, the mechanism of ethanol electrooxidation is investigated using the first principles method. It is found that CH3CHOH* is the key intermediate during ethanol electrooxidation and the activity of β-dehydrogenation is the rate determining factor that affects the completeness of ethanol oxidation. In addition, a series of transition metals (Ru, Rh, Pd, Os and Ir) are alloyed on the top layer of Pt(111) in order to analyze their effects. The elementary steps, α–, β–C–H bond and C–C bond dissociations, are calculated on these bimetallic M/Pt(111) surfaces and the formation potential of OH* from water dissociation is also calculated. We find that the active metals increase the activity of β-dehydrogenation but lower the OH* formation potential resulting in the active site being blocked. By considering both β-dehydrogenation and OH* formation, Ru, Os and Ir are identified to be unsuitable for the promotion of CO2 selectivity and only Rh is able to increase the selectivity of CO2 in DEFCs.

 Please wait while we load your content...
Please wait while we load your content...