Isomer-selective infrared spectroscopy of the cationic trimethylamine dimer to reveal its charge sharing and enhanced acidity of the methyl groups†
Abstract
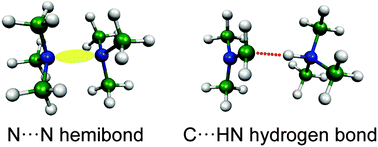
Infrared predissociation spectroscopy of the trimethylamine dimer cation generated by the vacuum-ultraviolet photoionization is isomer-selectively carried out by monitoring two main fragment channels, protonated trimethylamine and the trimethylamine monomer cation. The spectral carriers monitored by these two channels are assigned to different isomers of the trimethylamine dimer cation. One is the charge-shared (hemibond) structure, in which the positive charge is intermolecularly delocalized over the dimer through the interaction between the nonbonding orbitals of the nitrogen atoms. In the other isomer, a proton of a methyl group in the ionized moiety is intermolecularly transferred to the nitrogen atom of the neutral moiety and is shared between the carbon and nitrogen atoms. The latter isomer shows that the methyl groups of cationic trimethylamine are highly acidic. This example demonstrates characteristic properties of radical cations with alkyl groups.

 Please wait while we load your content...
Please wait while we load your content...