Iron near absorption edge X-ray spectroscopy at aqueous-membrane interfaces†
Abstract
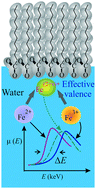
Employing synchrotron X-ray scattering, we systematically determine the absorption near-edge spectra (XANES) of iron in its ferrous (Fe2+) and ferric (Fe3+) states both as ions in aqueous solutions and as they bind to form a single layer to anionic templates that consist of carboxyl or phosphate groups at aqueous/vapor interfaces. While the XANES of bulk iron ions show that the electronic state and coordination of iron complexes in the bulk are isotropic, the interfacial bound ions show a signature of a broken inversion-symmetry environment. The XANES of Fe2+ and Fe3+ in the bulk possess distinct profiles however, upon binding they practically exhibit similar patterns. This indicates that both bound ions settle into a stable electronic and coordination configuration with an effective fractional valence (for example, Fe[2+ν]+, 0 < ν < 1) at charged organic templates. Such two dimensional properties may render interfacial iron, abundant in living organisms, a more efficient and versatile catalytic behavior.

 Please wait while we load your content...
Please wait while we load your content...