Aging effects of anatase TiO2 nanoparticles in Li-ion batteries
Abstract
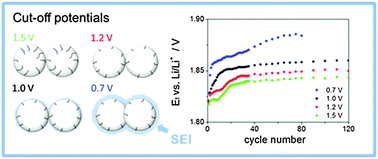
Anatase TiO2 nanoparticles with a diameter of 5 nm have been investigated as a negative intercalation electrode material for Li-ion batteries. The focus was on the stability upon cycling within four different potential ranges, namely from 1.5, 1.2, 1.0 and 0.7 V vs. Li/Li+ as the lower potential limit to 3.0 V vs. Li/Li+ as the upper potential limit. While a lower cut-off potential allows for a higher amount of charge stored, the irreversible processes induce a faster fading of the specific charge. Galvanostatic cycling (GC), electrochemical impedance spectroscopy (EIS) and scanning electron microscopy (SEM) experiments suggest that SEI formation has a negligible contribution to the irreversible processes. It appears more plausible that an irreversible degradation of the bulk phase occurs, leading to a decrease in the amount of active sites. Moreover, it has been observed that this degradation appears as an anodic shift of the thermodynamic potential of (de-)intercalation of Li-ions in the TiO2 structure. The shift is caused by a change in the activity of Li-ions in the solid phase, which is driven by changes in the ionic atmosphere of the crystal.

 Please wait while we load your content...
Please wait while we load your content...