Mechanistic and kinetic implications on the ORR on a Au(100) electrode: pH, temperature and H–D kinetic isotope effects
Abstract
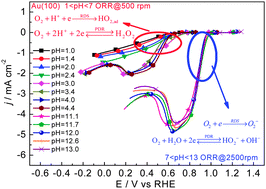
pH, temperature and H–D kinetic isotope effects (KIEs) on the ORR on Au(100) have been examined systematically using a hanging meniscus rotating disk electrode system. We found that for the cases with pH > 7, the ORR mainly goes through a 4-electron reduction to OH− at E > pzc (potential of zero charge) without any pH and H–D KIEs. When the pH at the electrode/electrolyte interface (pHs) is below 7, O2 only reduces to H2O2, its activity increases with pHs, and a H–D KIE of above 2 is observed in 0.1 M HClO4. According to the experimental results in acid solution, a mechanism with O2 + H+ + e → HO2,ad as the rate determining step followed by decoupled electron and proton transfer steps is proposed. The high activation barrier for O–O bond breaking and the fast oxidation of H2O2 or HO2− to O2 render the ORR observable only at potentials negative of the equilibrium potential (Eeq) of the redox of H2O2/O2 in acidic media or of HO2−/O2 in an alkaline environment. The apparent activation energy (Ea,app) for O2 reduction to H2O2 is ca. 35 ± 3 kJ mol−1 and to OH− is 60 ± 6 kJ mol−1, while the pre-exponential factor (A) for the former is ca. 3–6 orders of magnitude smaller than that of the latter. The lower activity for O2 reduction to H2O2 on Au(100) is attributed to the small pre-exponential factor.
- This article is part of the themed collection: Electrocatalysis - Fundamental Insights for Sustainable Energy

 Please wait while we load your content...
Please wait while we load your content...