Insight into the mechanism of nanodiamond catalysed decomposition of methane molecules†
Abstract
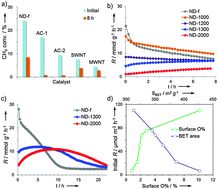
Nanodiamond can catalyze the decomposition of methane, and its initial rate is linearly dependent on the number of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– defective sites. Thermal treatment improves the ordering of surface atoms, resulting in an inferior activity but a more stable performance over a long period of time, and above 1300 °C few-layered graphene can be found.
CH– defective sites. Thermal treatment improves the ordering of surface atoms, resulting in an inferior activity but a more stable performance over a long period of time, and above 1300 °C few-layered graphene can be found.

 Please wait while we load your content...
Please wait while we load your content...