Experimental and theoretical studies of H2O oxidation by neutral Ti2O4,5 clusters under visible light irradiation†
Abstract
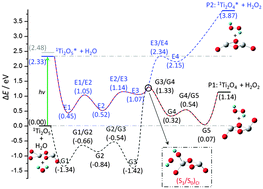
A new photo excitation fast flow reactor system is constructed and used to investigate reactions of neutral TimOn clusters with H2O under visible (532 nm) light irradiation. Single photon ionization at 118 nm (10.5 eV) is used to detect neutral cluster distributions through time of flight mass spectrometry. TimOn clusters are generated through laser ablation of a titanium target in the presence of 4% O2/He carrier gas. Association products Ti2O4(H2O) and Ti2O5(H2O) are observed for reactions of H2O and TimOn clusters without irradiation. Under 532 nm visible light irradiation of the fast flow reactor, only the Ti2O5(H2O) feature disappears. This light activated reaction suggests that visible radiation can induce chemistry for Ti2O5(H2O), but not for Ti2O4(H2O). Density functional theory (DFT) and time-dependent (TD) DFT calculations are performed to explore the ground and first excited state potential energy surfaces (PES) for the reaction Ti2O5 + H2O → Ti2O4 + H2O2. A high barrier (1.33 eV) and a thermodynamically unfavorable (1.14 eV) pathway are obtained on the ground state PES for the Ti2O5 + H2O reaction; the reaction is also thermodynamically unfavorable (1.54 eV) on the first singlet excited state PES. The reaction is proposed to occur on the ground state PES through a conical intersection ((S1/S0)CI), and to generate products Ti2O4 and H2O2 on the ground state PES. This mechanism is substantiated by a multi-reference ab initio calculation at the complete active space self-consistent field (CASSCF) level. The S0–S1 vertical excitation energy of Ti2O4 (3.66 eV) is much higher than the 532 nm photon energy (2.33 eV), suggesting this visible light driven reaction is unfavorable for the Ti2O4 cluster. The TDDFT calculated optical absorption spectra of Ti2O4 and Ti2O5 further indicate that Ti2O5 like structures on a titanium oxide surface are the active catalytic sites for visible light photo-catalytic oxidation of water.

 Please wait while we load your content...
Please wait while we load your content...