Spiropyran as a reusable chemosensor for selective colorimetric detection of aromatic thiols†
Abstract
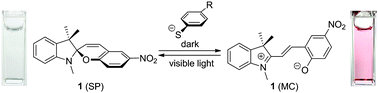
Design of optical molecular probes for selective detection of aromatic thiols has attracted much attention. Although several types of probes have been proposed, all of them exhibit colorimetric or fluorometric response via irreversible reaction with aromatic thiols and cannot be reused. Here we report that a spiropyran dye is the first example of a reusable chemosensor for aromatic thiols. A colorless spiropyran dye (1) dissolved in aqueous media containing aromatic thiols is selectively isomerized to the colored merocyanine form in the dark. In contrast, visible light irradiation of the merocyanine form promotes successful reversion to the colorless spirocyclic form. Kinetic absorption analysis and ab initio calculations of the transition states revealed that this colorimetric response in the dark is ascribed to the decrease in activation energy for isomerization via the nucleophilic interaction between the aromatic thiol and the olefinic carbon of the dye.

 Please wait while we load your content...
Please wait while we load your content...