Anisotropic charge-transfer effects in the asymmetric Fe(CN)5NO octahedron of sodium nitroprusside: a soft X-ray absorption spectroscopy study†
Abstract
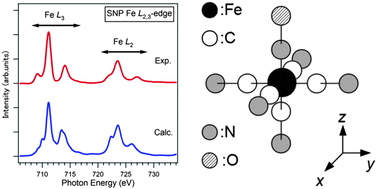
The electronic structure of Na2[Fe(CN)5NO]·2H2O (sodium nitroprusside: SNP) was investigated by using soft X-ray absorption (XA) spectroscopy. The Fe L2,3-edge XA spectrum of SNP exhibited distinct and very large satellite peaks for L3 and L2 regions, which is different from the spectra of hexacyanoferrates and the other iron compounds. A configuration-interaction full-multiplet calculation, in which the ligand molecular orbitals for the C4v symmetry were taken into account, revealed the Fe2+ low-spin state with very strong effects of metal-to-ligand charge-transfer from the Fe 3d to NO 2p orbitals.
![[u with combining overline]](https://www.rsc.org/images/entities/char_0075_0305.gif) suke
Nanba
suke
Nanba Please wait while we load your content...
Please wait while we load your content...